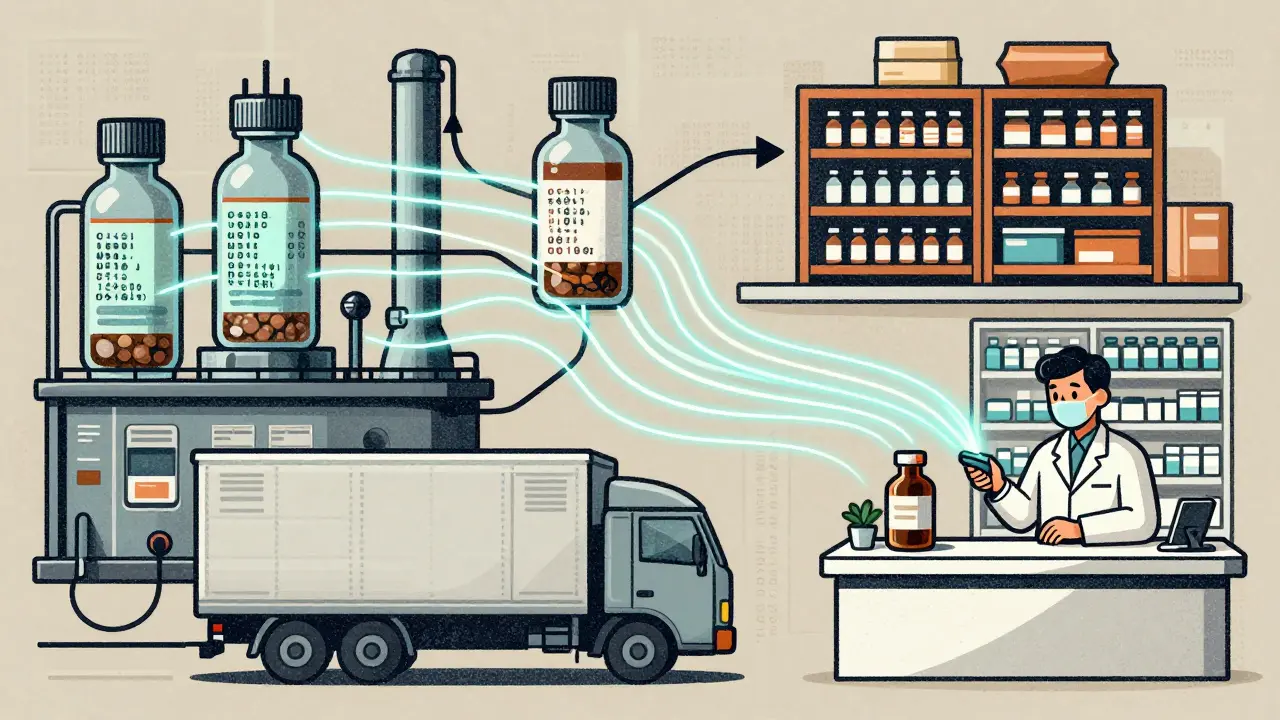
The DSCSA track-and-trace system is the U.S. government's solution to stop counterfeit drugs by requiring every prescription package to have a unique digital identifier. By 2024, all manufacturers, wholesalers, and pharmacies must verify drugs electronically to ensure safety.
If you receive the wrong medication from the pharmacy, act fast. Stop taking it, call your doctor, preserve evidence, and report the error. These steps can prevent harm and protect your legal rights.
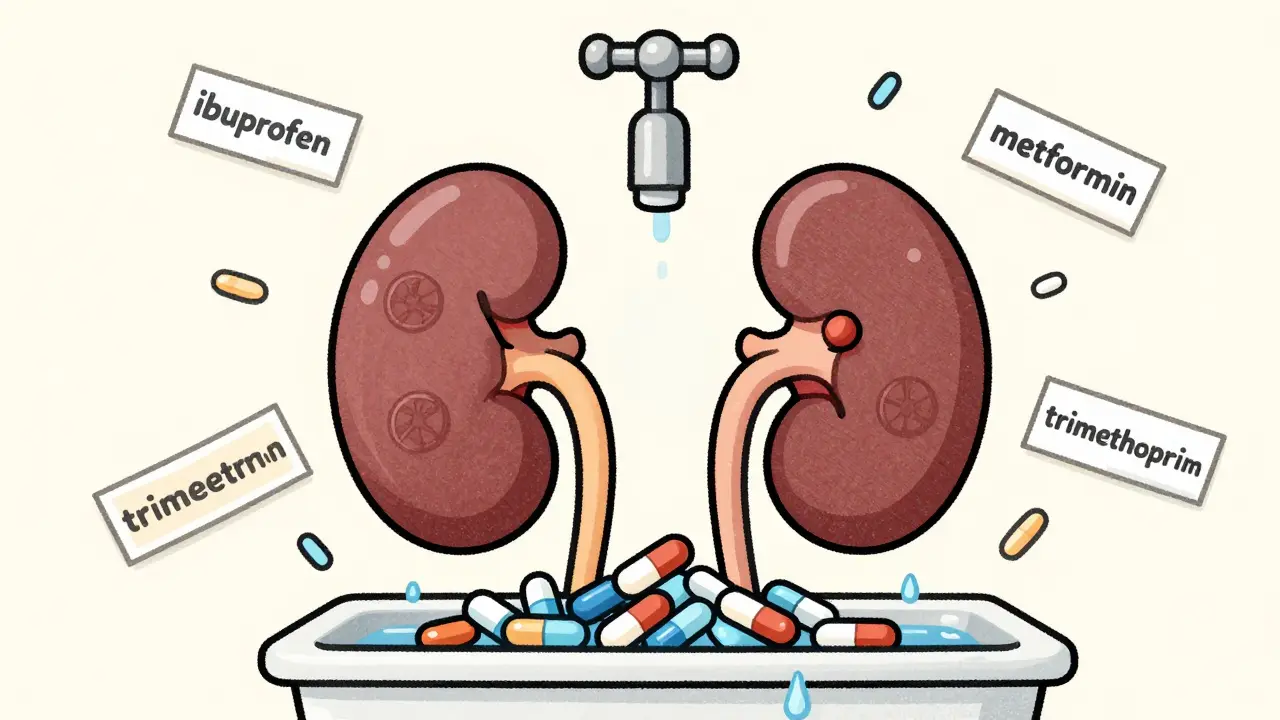
Kidney disease slows drug clearance, causing toxic buildup that can lead to organ failure. Learn which medications are most dangerous, how to spot risks, and what steps to take now to protect your kidneys.
Learn how healthcare providers navigate prior authorization to get generic medications approved by insurers, including documentation requirements, submission methods, approval timelines, and practical strategies to reduce delays and denials.
Simple lifestyle changes like walking, eating less salt, and sleeping better can reduce medication risks, lower doses, and sometimes eliminate the need for pills-backed by science and real patient results.
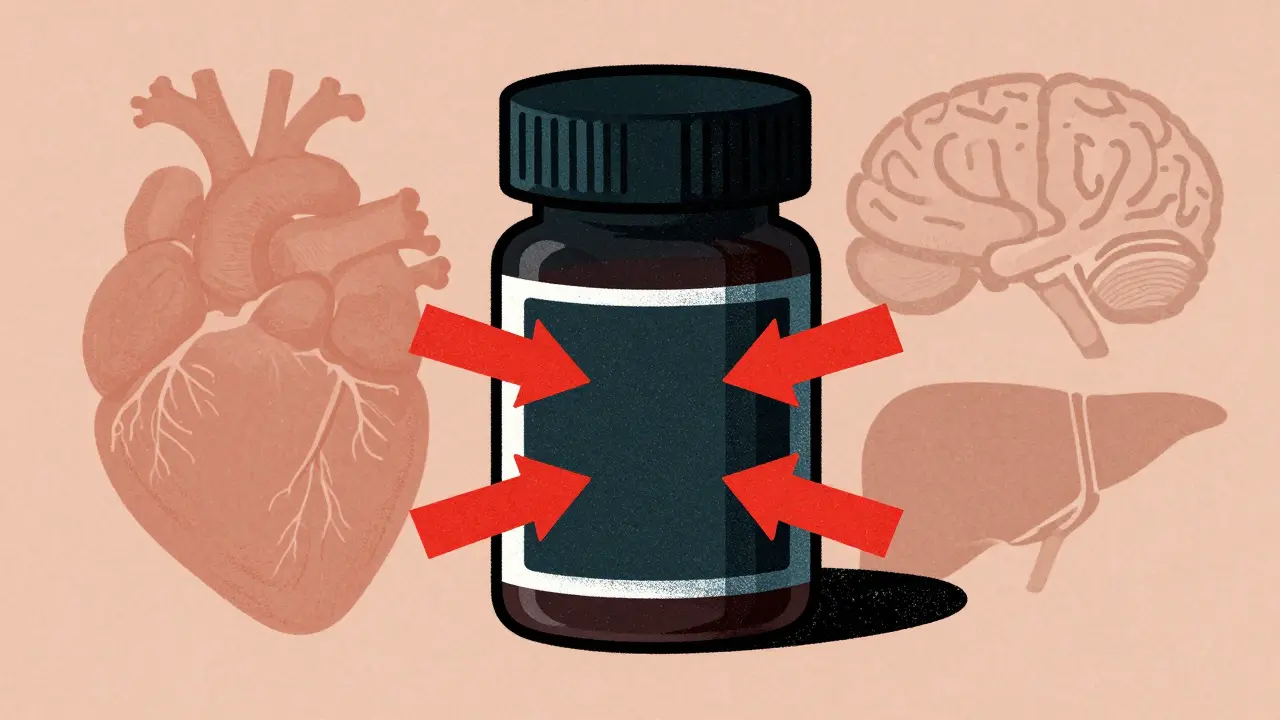
FDA black box warnings are the strongest safety alerts for prescription drugs, signaling life-threatening risks. Learn what they mean, which drugs carry them, and how to stay safe.
Type 1 diabetes is an autoimmune condition requiring lifelong insulin therapy. Learn the key symptoms, how it's diagnosed through blood tests and autoantibodies, and the latest insulin delivery options including pumps and CGMs.
Cardio burns calories fast during exercise, but strength training boosts your metabolism long after you stop. For real, lasting weight loss, you need both - not one or the other.
Calibration and validation are essential for manufacturing quality, ensuring equipment accuracy and reliability. Learn how ISO 13485, FDA, and CLIA requirements work, common pitfalls, and how to implement a compliant, cost-effective system.
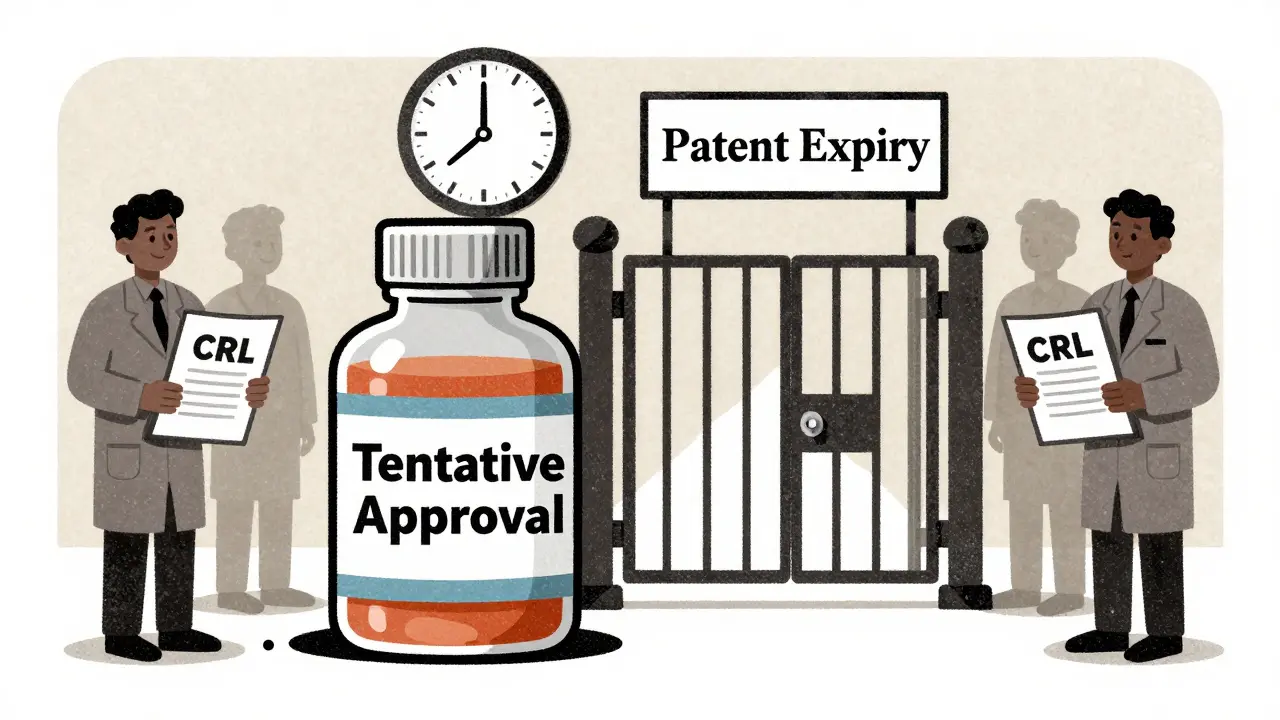
Tentative FDA approval for generics means the drug is scientifically ready - but still can't be sold due to patents, lawsuits, or manufacturing issues. Learn why most generics sit idle for over a year after approval.