Institutional Programs in Healthcare: What They Are and Why They Matter
When we talk about institutional programs, structured systems within hospitals, pharmacies, and health agencies that manage drug use, safety, and distribution. Also known as healthcare operational protocols, these programs are the backbone of how medications move from manufacturers to patients—especially in high-stakes settings like emergency rooms and long-term care units. These aren’t just paperwork or policies. They’re the reason your hospital pharmacy can still get life-saving injectables during a shortage, why your generic pill looks different but works the same, and why your doctor knows whether a new drug is truly safe for your condition.
Hospital pharmacy, the frontline unit responsible for stocking, dispensing, and monitoring medications in clinical settings runs on institutional programs that track everything from expiration dates to drug interactions. For example, 60% of the drugs in short supply are sterile injectables—critical for surgeries, cancer treatments, and ICU care. Without institutional programs that prioritize inventory, track suppliers, and flag risks, patients would face dangerous delays. These same programs also handle how FDA generic approval, the process by which the U.S. Food and Drug Administration evaluates whether a generic drug is as safe and effective as the brand-name version works behind the scenes. When a manufacturer changes its production line, the FDA doesn’t just accept it—they re-evaluate the whole chemistry, packaging, and stability. Institutional programs make sure those changes don’t slip through the cracks.
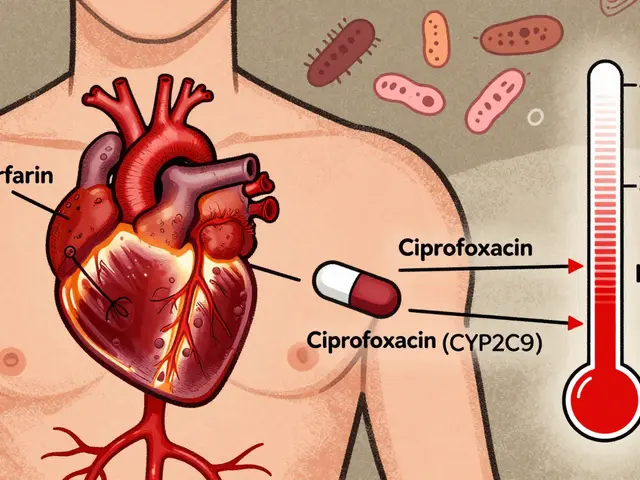
And it’s not just about getting the right drug. medication safety, the system of practices designed to prevent harm from drugs during prescribing, dispensing, or taking depends on institutional programs to catch errors before they happen. Think about how many people mix up pills, skip doses, or don’t know what their label means. Institutional programs train staff, automate alerts, and even use home health services to double-check doses for seniors. They’re why you can safely split a pill to save money, why your pharmacist can explain why a generic works just as well, and why you’re not left guessing if alcohol will mess with your blood thinner.
Behind every safe medication experience is a network of institutional programs—some visible, most hidden. They connect drug supply chain, the full path a medication takes from raw ingredients to the patient’s hand, including manufacturing, distribution, and regulation to real-world outcomes. When a generic drug gets approved faster under new EU rules, or when a hospital tracks adverse events using the FDA’s FAERS database, it’s because someone built a system to make it happen. These aren’t abstract ideas. They’re the reason you didn’t get a fake fentanyl pill, why your clozapine dose didn’t crash when you quit smoking, and why your dentist didn’t make you stop your blood thinner before a cleaning.
What you’ll find below isn’t just a list of articles. It’s a window into how these systems work—where they succeed, where they break, and how you can use that knowledge to protect yourself. From how hospitals handle drug shortages to how patients are taught to trust generics, every post here ties back to the real-world impact of institutional programs.