Hatch-Waxman Act: How Generic Drugs Got Approved and Changed Healthcare
When you pick up a generic pill at the pharmacy, you’re seeing the result of the Hatch-Waxman Act, a 1984 U.S. law that balanced drug innovation with affordable access by creating a faster path for generic medications. Also known as the Drug Price Competition and Patent Term Restoration Act, it’s the reason you can buy the same medicine as a brand-name drug for a fraction of the cost. Before this law, companies spent years and millions just to prove a generic version worked — even when the original patent had expired. The Hatch-Waxman Act fixed that by letting generic makers rely on the brand’s safety data, as long as they showed their version was bioequivalent. That single change opened the floodgates for affordable medicines.
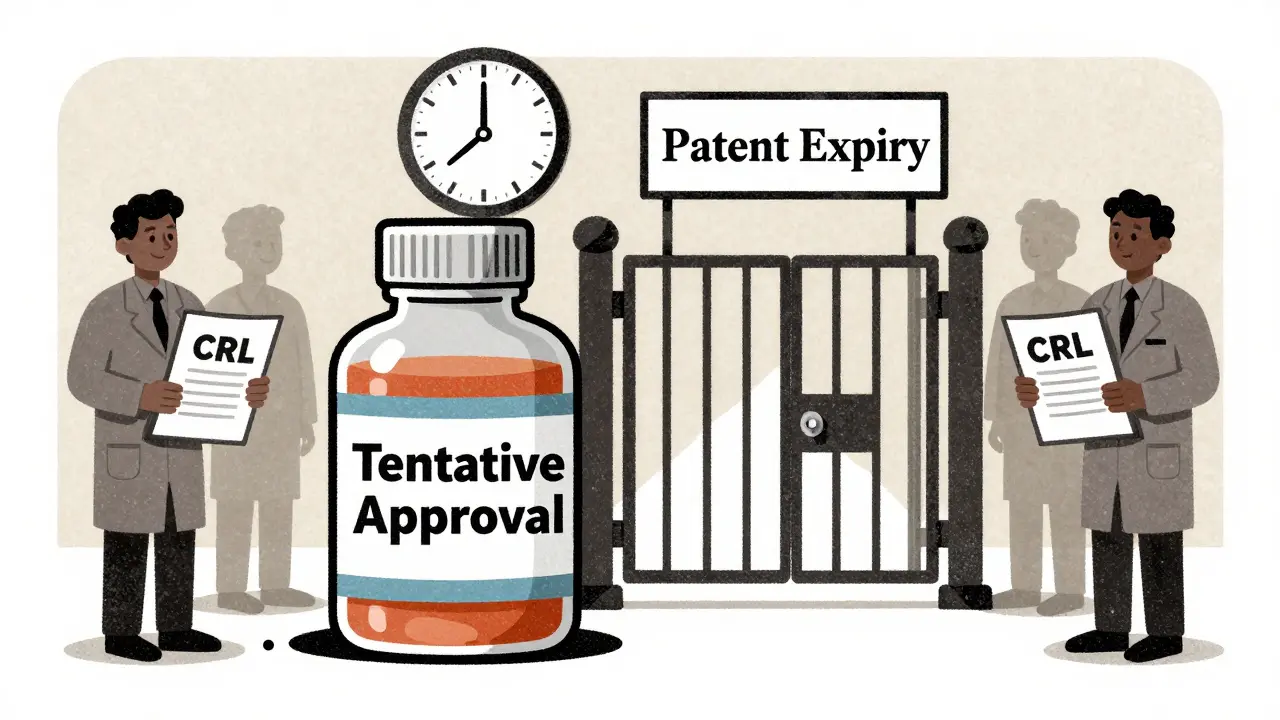
The law didn’t just help patients — it reshaped how drug companies think about patents. It gave brand-name makers up to five extra years of market exclusivity to make up for time lost during FDA review. At the same time, it let generic companies challenge weak patents and file for approval as soon as the patent expired or was found invalid. This created a race — not just to make drugs cheaper, but to be first to market. The first generic to file often gets 180 days of exclusive rights, which is why you sometimes see one generic version hit the shelves before others. This system, built on ANDA, the Abbreviated New Drug Application process used by generic manufacturers to gain FDA approval, became the backbone of U.S. drug supply. Today, over 90% of prescriptions in America are filled with generics — thanks largely to this law.
It also created the framework for how we handle drug competition, the dynamic between brand-name and generic manufacturers that drives down prices and increases access. When a patent runs out, the market shifts. Manufacturers rush to file applications. Pharmacies switch stock. Prescribers start recommending generics. But the law didn’t just encourage competition — it tried to prevent abuse. It forced companies to list patents in the FDA’s Orange Book, so generics knew what they were up against. It also gave generics legal protection if they challenged a patent in good faith. That’s why you see lawsuits over patents — they’re not just legal battles, they’re part of the system designed to keep prices low.
Today, the Hatch-Waxman Act still controls how most generics get approved. But it’s not perfect. Some companies game the system by making tiny changes to extend exclusivity. Others delay generics with legal tactics. Still, the core idea holds: patients win when competition is allowed. The posts below show how this law ripples through real-world issues — from manufacturing changes that trigger FDA re-evaluation, to how providers and patients navigate generic substitutions, to why some drug shortages happen when patent cliffs hit. Whether you’re taking a generic statin, an antibiotic, or a thyroid pill, you’re living with the legacy of this law. And understanding it helps you ask the right questions — and get the best care at the best price.