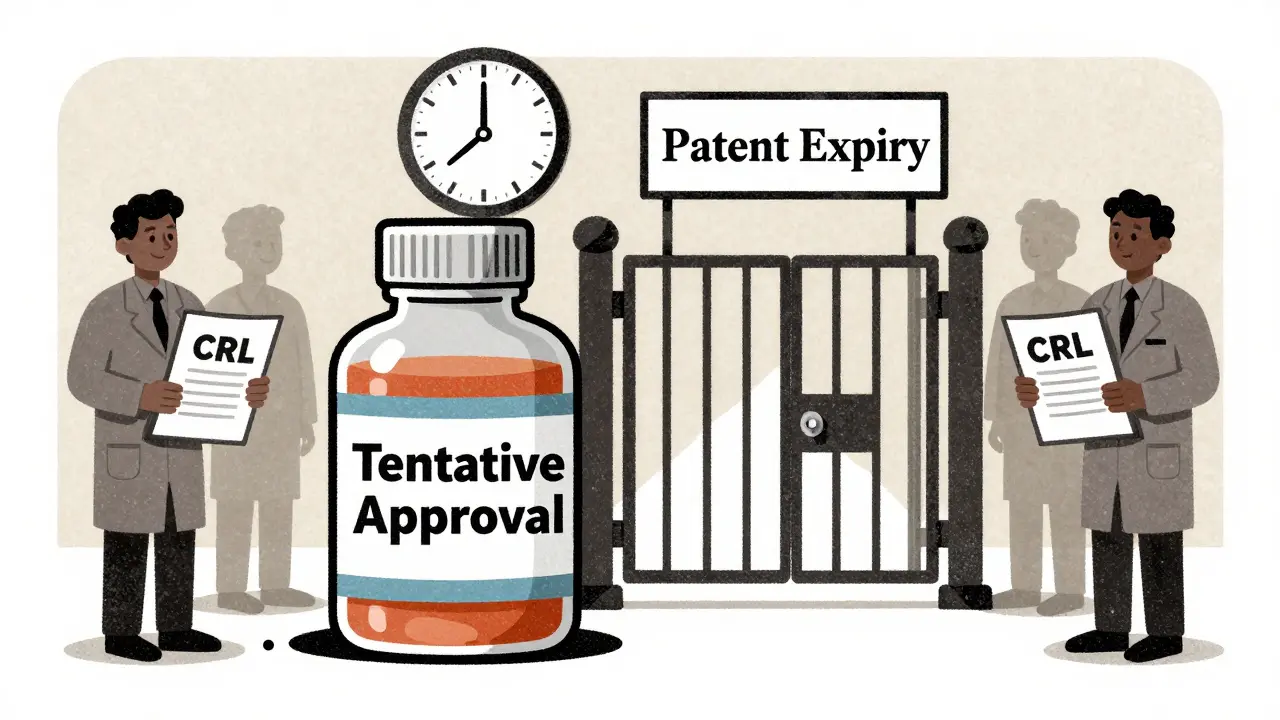
Tentative FDA approval for generics means the drug is scientifically ready - but still can't be sold due to patents, lawsuits, or manufacturing issues. Learn why most generics sit idle for over a year after approval.
13 January 2026 by Tristan McCarthy
10