Pharmaceutical inspection: what it means and why it matters
Many pharmacies and suppliers look professional, but a proper pharmaceutical inspection can reveal quality gaps you won’t spot at first glance. If you buy meds online or work with suppliers, knowing what inspectors check helps you avoid fake drugs, storage errors, and dangerous mistakes.
At a basic level, a pharmaceutical inspection confirms that drugs are made, stored, transported, and sold the right way. Inspectors focus on safety, traceability, and whether a business follows law and good manufacturing or distribution practice (GMP/GDP). For you, that translates into getting the product you expect — the right drug, strength, and packing — every time.
What inspectors look for (plain and practical)
Here are the common checks inspectors run — and the simple things you can look for too:
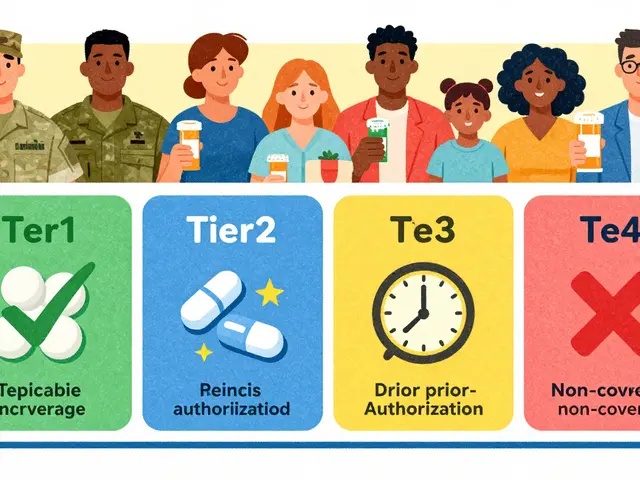
- Licenses and certificates: inspectors verify pharmacy or manufacturer licenses and quality certificates. You can ask an online pharmacy for their licensing info and check it on the regulator’s website (MHRA in the UK, FDA in the US, TGA in Australia).
- Storage and temperature control: medicines often need specific temps. Inspectors check fridges, temperature logs, and alarms. On a site, look for mentions of cold-chain shipping and real-time tracking for temperature-sensitive items.
- Batch records and traceability: every batch should be traceable from raw material to finished product. Ask for batch numbers and expiry dates on every order and keep them for reference.
- Packaging and labeling: correct drug name, dosage, lot number, expiry, manufacturer info, and safety leaflets. Scruffy labels, unusual fonts, or missing leaflets are red flags.
- Quality testing and sampling: inspectors sample products for lab testing (identity, potency, impurities). Reputable sellers can point to independent lab checks on request.
Quick checks you can do before buying
- Verify the pharmacy: check the site’s registered business name, physical address, and license number on the national regulator site. If it’s hard to find, don’t buy.
- Read product photos closely: compare packaging to the maker’s official images. Watch for spelling mistakes, poor print, or different logos.
- Ask for a prescription policy and pharmacist contact: legitimate online pharmacies require a valid prescription for prescription-only meds and have a pharmacist available to answer questions.
- Check reviews and third-party mentions: look beyond testimonials on the site. Search for news, forum threads, and regulator alerts about the seller.
- Keep records: save order confirmations, photos on arrival (including packaging and labels), and batch numbers. That makes reporting a problem much easier.
Pharmaceutical inspection isn’t just for regulators — knowing the basics protects your health and wallet. When a seller can’t show licensing, batch info, or proper storage, treat it as a hard stop. Safe meds start with transparency and traceability; if those are missing, look elsewhere.