Pharmacovigilance: Understanding Drug Safety and Real-World Side Effects
When you take a pill, you’re trusting that it’s been tested for safety—but what happens after it hits the market? That’s where pharmacovigilance, the science of monitoring drug safety after approval. Also known as drug safety surveillance, it’s the quiet system that catches problems clinical trials miss. Big studies involve thousands of people. Real life involves millions. And in real life, people take multiple drugs at once, have different genetics, drink alcohol, smoke, or forget to tell their doctor about their herbal supplements. That’s where things go wrong—and where pharmacovigilance steps in.
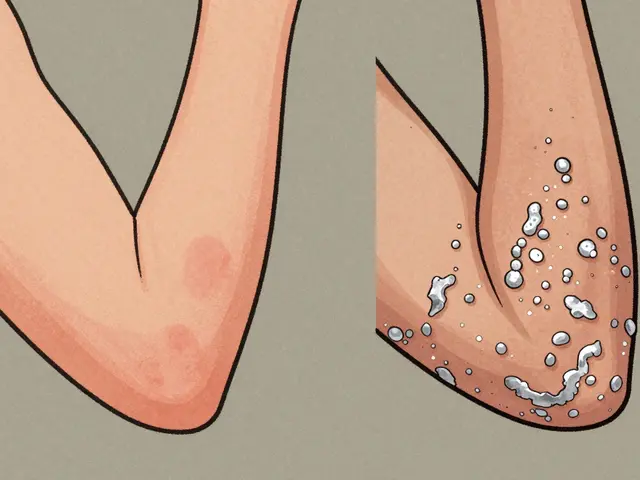
Pharmacovigilance isn’t just about counting complaints. It’s about spotting patterns. For example, a drug might seem safe in trials, but if 500 people on the same medication suddenly develop liver issues months later, regulators need to know. That’s how we learned smoking cuts clozapine levels in half, or how alcohol spikes INR in people on warfarin. These aren’t theoretical risks—they’re real, documented events tracked through reports from doctors, patients, and pharmacies. The adverse drug reactions, harmful and unintended responses to medications. Also known as ADR, they’re the core data source for pharmacovigilance. And they’re not the same as side effects. Side effects are expected, like drowsiness from antihistamines. Adverse reactions are dangerous surprises—like a rash turning into a life-threatening skin condition. Confusing the two can lead to bad decisions. That’s why pharmacovigilance separates the noise from the real threats.
It’s also why you see posts about generic substitutions, drug interactions with protein shakes, or how fentanyl hides in fake pills. These aren’t random topics—they’re all part of the same safety net. Pharmacovigilance tracks how generics behave in real patients, how food changes absorption, and how counterfeit drugs slip through cracks. It’s the reason labels now warn about CYP3A4 interactions or sulfonamide cross-reactivity. These aren’t just fine print—they’re lessons learned from people who got hurt before the warnings existed.
What you’ll find here isn’t theory. It’s stories from providers who’ve seen generics fail, patients who nearly overdosed on fake Ativan, or people who didn’t know their morning protein shake was blocking their thyroid meds. These are the kinds of risks pharmacovigilance catches—before they become headlines. If you take any medication regularly, this isn’t just background noise. It’s your safety layer.