When you pick up a generic pill, you expect it to work just like the brand-name version. But behind the scenes, manufacturing flaws can make those pills unsafe-even if they look identical. Between 2019 and 2023, generic drugs had 3.2 times more manufacturing quality issues than branded ones, according to FDA inspection data. These aren’t rare mistakes. They’re systemic problems rooted in cost-cutting, outdated equipment, and weak oversight.
What Exactly Goes Wrong in Generic Drug Manufacturing?
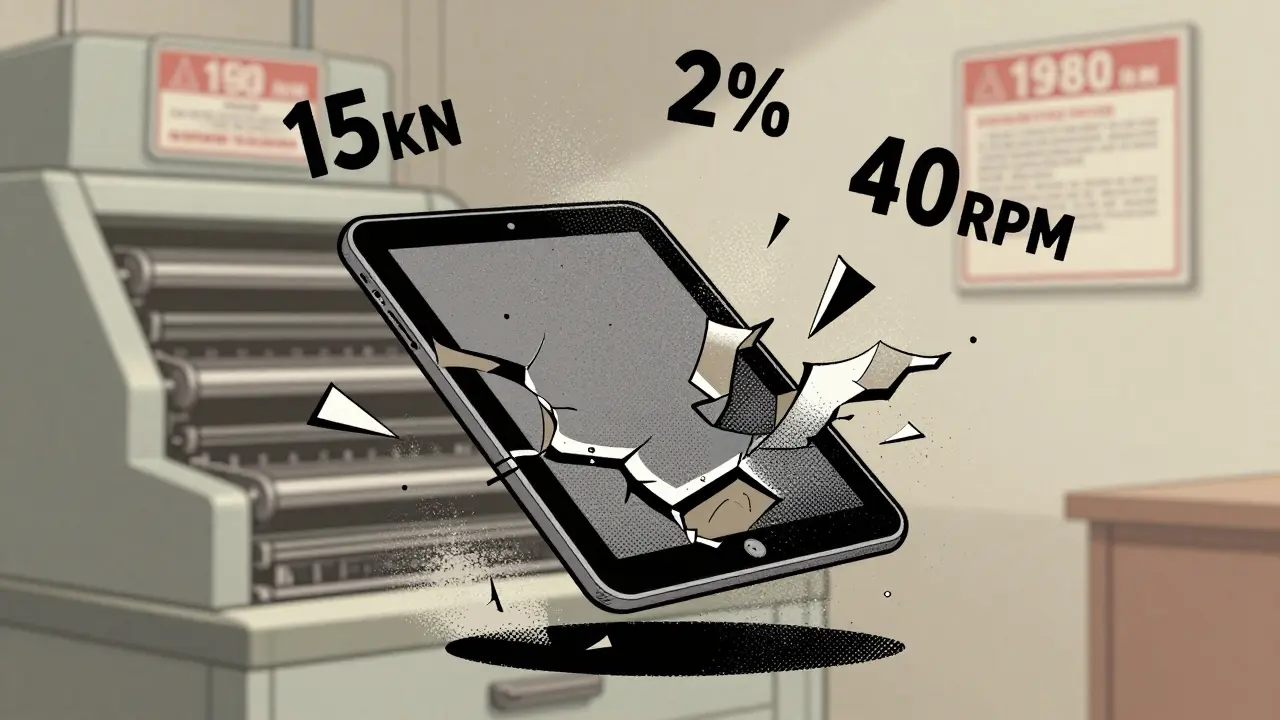
Generic drug makers don’t just copy formulas-they copy production lines. And many of those lines are decades old. A tablet press from the 1980s doesn’t have the sensors or precision of modern machines. That’s why defects like capping and lamination happen so often.Capping is when the top or bottom of a tablet splits off during handling. It happens when compression force exceeds 15 kN and the moisture content in the powder is below 2%. This is common in hydrophobic drugs like ibuprofen or metformin. Lamination is when layers peel apart inside the tablet, usually because the turret speed goes above 40 rotations per minute and pre-compression isn’t set right.
Then there’s sticking. When the active ingredient melts slightly under heat and pressure, it glues itself to the punch heads. This slows production and causes inconsistent dosing. If your API has a melting point below 120°C and the humidity hits 4%, sticking spikes by 300-500 N of ejection force. That’s not a glitch-it’s a design flaw in the process.
Weight variation is even more dangerous. The USP <905> standard says no more than 5% deviation from target weight. But when granule flow rates drop below 0.5 g/s, you get batches where some pills have 10% too much drug and others have 10% too little. That’s not just a quality issue-it’s a risk of overdose or treatment failure. In 2023, 12.7% of affected batches failed dose uniformity tests.
Why Are Generic Drugs More Prone to These Problems?
Branded drug companies spend 15-18% of their production budget on quality control. Generic manufacturers? Only 8-10%. Why? Because they’re fighting over pennies. Generic drugs make up 90% of prescriptions in the U.S., but only 23% of total drug spending. That means every milligram saved on quality adds to the bottom line.Many generic makers share facilities with multiple products. One batch of antibiotics might be made right after a steroid. Cross-contamination isn’t rare-it’s expected in some plants. The FDA found 57% of generic manufacturing sites failed inspections in 2023, compared to just 28% for branded sites.
Complex formulations are the worst offenders. Inhalers have an 18.2% defect rate because getting the right particle size and dose consistency is incredibly hard. Modified-release tablets? 14.7%. Even simple immediate-release tablets aren’t safe-9.3% still have defects. But the real danger is in injectables. Sterile products account for 78% of drug shortages tied to quality, mostly because of particulate contamination or failed sterilization.
How Do These Defects Affect Patients?
Patients don’t always know when they’ve been given a faulty pill. But pharmacists do. A 2023 survey of 1,247 U.S. pharmacists found 68% had seen quality issues in the past year. Forty-two percent reported patients complaining about tablets that crumbled, cracked, or had weird color spots. Twenty-nine percent said patients felt the generic didn’t work the same as before.One Reddit user posted about a batch of metformin ER that turned to dust during dispensing. Another described how different levothyroxine generics made patients feel anxious or fatigued-even though they were from the same manufacturer. These aren’t anecdotes. In 2023, the FDA’s MedWatch system received 1,842 reports of adverse events linked to visible tablet defects. Over 300 involved chipping, discoloration, or cracking.
Hospitals are catching on. In 2023, 17.3% of generic substitution requests were rejected because of quality concerns. Nearly 10% of those cases led to permanent switches back to brand-name drugs. That’s not just inconvenience-it’s added cost and risk for patients who can’t afford the brand.

What’s Being Done to Fix This?
The FDA’s 2022 Quality by Design (QbD) guidelines pushed manufacturers to map out their entire production process and define safe operating ranges. Instead of guessing what works, they now have to prove it-using real-time data. Some companies are responding.Continuous manufacturing is replacing batch processing in 47 generic plants. This reduces defect rates by 65%. Real-time weight monitors now reject pills outside ±5% of target weight at 1,200 tablets per minute. Automated visual inspection systems spot defects as small as 0.1 mm with 98% accuracy-down from 30% error rates with human inspectors.
AI is starting to make a difference. Pilot programs at Sandoz and Dr. Reddy’s use machine learning to predict defects before they happen. These systems catch 92% of flaws, compared to 78% with traditional methods. The FDA’s Emerging Technology Program is helping small manufacturers adopt these tools with grants and technical support.
The 2024 Drug Supply Chain Security Act now requires track-and-trace for high-risk generics. Early results show a 22% drop in counterfeit-related quality issues. That’s progress-but it’s not enough.
Why This Isn’t Getting Better Fast Enough
The problem isn’t just outdated machines. It’s money. McKinsey & Company estimates it would take $28.7 billion to upgrade all U.S. generic manufacturing facilities to modern standards. The industry spends $1.2 billion a year. That’s a $27.5 billion gap.Some companies are thriving. Teva reported a 0.8% batch rejection rate in 2023. Smaller manufacturers? 3.2%. The top 10 generic makers now control 58% of the market. But that doesn’t mean safety is improving across the board. Many small players can’t afford upgrades. They’re stuck making pills with 1990s tech and 2020s demands.
Regulators are catching on. The EMA rejected 37% of generic applications in 2023 due to manufacturing defects-up from 29% in 2019. The FDA issued 42% of its warning letters to generic makers for quality violations. Yet, price pressure hasn’t eased. If anything, it’s getting worse.

What Patients and Providers Can Do
If you notice a generic pill looks different-wrong color, odd shape, crumbles easily-don’t ignore it. Talk to your pharmacist. Ask if it’s the same batch as before. Check the lot number. Report it to the FDA’s MedWatch system.Hospitals and pharmacies should track rejection rates by manufacturer. If one generic brand consistently fails inspections, switch to another. Don’t assume all generics are equal. They’re not.
For prescribers, consider documenting quality concerns in patient charts. If a patient reports a change in how a generic works, it might not be in their head-it could be in the pill.
Long-term, we need policy changes. Incentives for modernization. Penalties for repeated violations. Transparency in inspection results. Right now, the system rewards the cheapest supplier, not the safest one. That has to change.
What are the most common physical defects in generic tablets?
The most frequent defects include capping (tablet splitting at the top or bottom), lamination (layer separation), sticking (material adhering to machine parts), mottling (uneven color), and weight variation (pills too heavy or too light). These are often caused by outdated equipment, poor moisture control, or incorrect compression settings.
Are generic drugs less safe than brand-name drugs?
Not inherently-but manufacturing quality is far more inconsistent. Generic drugs are required to be bioequivalent, but defects in production can lead to underdosing, overdosing, or contamination. FDA data shows generic manufacturers have 3.2 times more quality-related inspection failures than branded ones.
Can a defective generic drug still be bioequivalent?
Yes. Bioequivalence tests measure how much drug enters the bloodstream, not tablet integrity. A cracked tablet or one with uneven drug distribution can still pass a blood test but deliver inconsistent doses. That’s why physical defects matter-even if the drug is technically "bioequivalent."
How can I tell if my generic medication has a defect?
Look for visible signs: cracking, chipping, unusual color spots, powdery residue, or pills that crumble when handled. If the pill looks different from your last refill-even if the name is the same-ask your pharmacist about the manufacturer and lot number. Report it if something seems off.
Why aren’t more generic manufacturers upgrading their equipment?
Because it’s expensive. Upgrading a single production line can cost millions. With profit margins often under 5%, many companies can’t justify the investment. Price competition, not lack of knowledge, is the main barrier. Only large manufacturers like Teva or Sandoz have the resources to modernize fully.
What’s Next for Generic Drug Safety?
The FDA’s 2024-2027 plan aims to cut quality-related shortages by 30%. That’s a start. But without real funding, it’s just a goal. AI-driven quality control, continuous manufacturing, and mandatory transparency are the tools we have. What’s missing is the will to use them.Patients deserve safe, reliable medications-not a lottery based on which factory made their pill. Until manufacturers are rewarded for quality, not just low cost, these defects won’t disappear. They’ll just keep showing up in new batches, new drugs, and new patients.
Thomas Varner
January 18, 2026 AT 18:43
I've had pills crumble in my hand before. Not kidding. One batch of metformin looked like it was made from crushed chalk. Took it anyway because I couldn't afford the brand. My blood sugar went haywire for two weeks. Now I check every pill like it's a bomb. 🤷♂️
Renee Stringer
January 19, 2026 AT 13:09
This is what happens when you treat healthcare like a commodity. People are not widgets. Lives are not line items on a balance sheet. And yet, here we are.
Jacob Cathro
January 21, 2026 AT 03:07
so like... the FDA is just letting these sketchy factories pump out pills that fall apart?? bro. i mean. they literally have machines that can detect a hair on a potato. but they can't stop a tablet from capping? wtf is this? #capitalism #pharmaconspiracy
Manoj Kumar Billigunta
January 21, 2026 AT 23:56
Many of us in developing countries rely on generics because that's all we can afford. But this is heartbreaking. If even basic quality control is ignored, then we're trading safety for affordability. We need global standards-not just American ones.
Andy Thompson
January 23, 2026 AT 13:43
THEY'RE DOING THIS ON PURPOSE. You think it's an accident? Nah. It's a plan. Big Pharma wants you hooked on their $500 brand pills. They let the generics be crap so you panic and go back. It's a scam. The FDA? In on it. #BigPharmaLies #WakeUpAmerica 🇺🇸
sagar sanadi
January 24, 2026 AT 14:02
Wait so you're telling me the same exact pill made in India costs $2 but might kill you, while the same pill made in Germany costs $50 and works fine? So... the problem isn't generics. It's just that Americans are too lazy to pay for quality. Face it.
kumar kc
January 25, 2026 AT 21:11
This is why you shouldn't trust anything made for profit. Ever.
Emily Leigh
January 27, 2026 AT 06:03
I mean... if your pill looks like it was rolled in a dryer with gravel, maybe it's time to stop pretending this system works. We're not just talking about a few bad batches. We're talking about an entire industry built on ignoring physics and biology because margins are tight. And yet... we still act surprised when people get sick?
Carolyn Rose Meszaros
January 27, 2026 AT 19:25
I work in a pharmacy and I see this every week. One day the metformin is fine, next day it’s crumbling like a cookie. We have to tell patients to handle them like eggs. It’s exhausting. And no one listens until someone ends up in the ER. 😔
Greg Robertson
January 29, 2026 AT 06:41
I get that generics save money. But if your medication doesn’t hold together, you’re not saving anything-you’re just gambling. Maybe we should start labeling them with risk levels instead of just price tags.
Crystal August
January 29, 2026 AT 15:37
I’ve been on the same generic for 8 years. Last month, I got a new bottle and it tasted like plastic. I threw it out. My doctor laughed. Said it was "probably just the coating." I’m not buying that anymore. This isn’t normal. This is negligence.
Nadia Watson
January 29, 2026 AT 22:01
It is deeply concerning that systemic quality control failures persist in a sector responsible for human health. The disparity between inspection failure rates-57% for generics versus 28% for branded-is not merely statistical; it reflects a moral failure in regulatory prioritization. We must demand transparency, not just efficiency.
Courtney Carra
January 31, 2026 AT 18:07
We’re all just trying to survive. But if the pill you swallow to stay alive is literally falling apart... isn’t that the ultimate irony? We’re treating illness like a math problem. But bodies aren’t equations. They’re fragile. And we’re treating them like disposable packaging.
thomas wall
February 2, 2026 AT 01:55
The erosion of pharmaceutical standards is not an accident-it is the inevitable consequence of deregulation, privatization, and the commodification of life. The West has outsourced its moral responsibility to the lowest bidder. This is not progress. This is decay.
Shane McGriff
February 2, 2026 AT 17:20
I’ve been a nurse for 18 years. I’ve seen patients crash because their levothyroxine didn’t work. They didn’t know why. We didn’t know why. Then we checked the lot number. Same manufacturer. Different batch. Same problem. We switched them back to brand. They stabilized in 48 hours. This isn’t placebo. It’s physics. And someone’s ignoring it.